Protein Critical For Development In Fruit Flies Found To Aid Healing Of Cuts And Wounds In Mammals
June 13, 2003
By: Sherry Seethaler
Biologists at the University of California, San Diego have determined that a protein essential for the normal embryonic development of fruit flies is also used by mammals to assist in the timely healing of cuts and lacerations.
Their discovery, detailed in the June 3 issue of the journal Developmental Cell, provides new insight for scientists into the molecular mechanisms responsible for wound healing in humans and may one day lead to the design of new drugs for individuals whose healing is compromised.
Improving wound healing is particularly important for burn victims and others with slow healing skin lesions like those associated with diabetes. Diabetes now affects more than 17 million Americans and the most prevalent form—type 2, or insulin-resistant diabetes—continues to skyrocket. The U.S. Centers for Disease Control and Prevention estimate that the number of diagnosed cases will increase 165 percent by 2050, as the number of overweight adults and children climbs in the United States.
Geneticists have long been aware of the role played by “c-Jun”—a protein called a “transcription factor” because it turns genes on and off— in bringing cells together during the embryonic development of Drosophila, the fruit flies commonly used in genetic research. For many years, Drosophila biologists have even suspected that the same biochemical pathways involved in a process called dorsal closure in the development of fly embryos were involved in wound healing in mammals. During dorsal closure in Drosophila, sheets of cells come together and fuse along the dorsal midline. The process looks similar to the way sheets of skin cells come together during wound healing in mammals.
“However, healing skin is a very different thing than putting together sheets of cells in making a fly,” says Randall S. Johnson, an associate professor of biology at UCSD who headed the research team. “So we really weren’t sure what to expect when we began our study. But we eventually demonstrated in our experiments that the loss of this protein in the skin of mice causes cells to bunch up at the leading edge of a wound, much like water rushing into a curb.”
“If we can eventually design drugs to promote this chemical pathway,” he adds, “surgeons will be able to improve the recovery of their patients and the increasing number of diabetics in this country will be able to improve their ability to heal from cuts and lacerations.”
The researchers in the Johnson lab were assisted by Kit Pogliano, an associate professor of biology at USCD; Steven K. Hanks of Vanderbilt University School of Medicine; Katie Nason and Jeffrey M. Arbeit from UC San Francisco School of Medicine and Ronald M. Wisdom from UC Davis.
While geneticists previously knew that in Drosophila c-Jun is needed to initiate the movement of cells in dorsal closure, a major difference between wound healing in mammals and dorsal closure in Drosophila is that, in wound healing, skin cells that were together become separated as a result of injury. In dorsal closure in Drosophila, cells that were never together receive some signal that makes them migrate towards each other. To see if c-Jun played a role in wound healing in mammals, Cindy Gustafson-Brown and Guochun Li, postdoctoral fellows in Johnson’s lab, produced a strain of mice that lacked the c-Jun protein in skin cells.
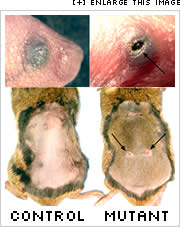
Surprisingly, these mice were born with open eyes. Mice, like kittens and puppies, are usually born with closed eyes. Eyelid closure occurs in the later stages of embryonic development when skin cells rapidly proliferate and migrate across the eye. Initially the researchers were quite puzzled by the open-eye defect in the mice.
“We spent a long time scratching our heads and wondering, what does that mean?” says Johnson. “It was when we also saw the pattern of migration in the wound that it became clearer.”
The wounds of mice lacking c-Jun protein in skin cells healed more slowly than the wounds of wild-type mice. Even more revealing were microscopic images of skin samples near the wound. Wound healing is a multi-stage process that begins with the formation of a fibrin clot, the initial scab, and ends with the migration and proliferation of skin cells through the fibrin clot to close the wound. However, in the mice lacking c-Jun, skin cells seemed to proliferate properly, but bunched up at the edge of the wound and failed to move across.
“The clear malformation at the leading edge indicates that this is the target zone for the function of this protein during wound healing,” says Johnson.
The researchers also found that the skin cells at the edge of the eye in mutant mice were bunching up and stalling, just as they did at the edge of the wound. To understand the molecular reason for these defects, the researchers took skin tissue samples from the wound and the edge of the eye in mutant and normal mice, then stained them with markers to certain proteins known to be involved in cell communication in Drosophila. They found decreased levels of several of these proteins in the leading edge skin cells along the wound and eye in the mutant mice. The cells from the mutant mice also had defects in the cytoskeleton, the cell’s support structure, which is important for cell mobility.
The protein c-Jun is a member of a family of proteins in which many proteins have overlapping functions. This may explain why the mutant mice did not have more severe skin defects, says Li, the first author of the study. “C-jun might be specifically required when the balance of skin cell proliferation and differentiation are rapidly and temporally altered, such as in wound healing or growth of tumors.”
In addition to the implications of these findings for drug design to promote wound healing, Li points out, “Our mutant mice give us a terrific tool to study the function of this protein in tumor formation and in other skin diseases.”
