Biologists Discover New Strategy to Treat Central Nervous System Injury
April 11, 2016
By Kim McDonald
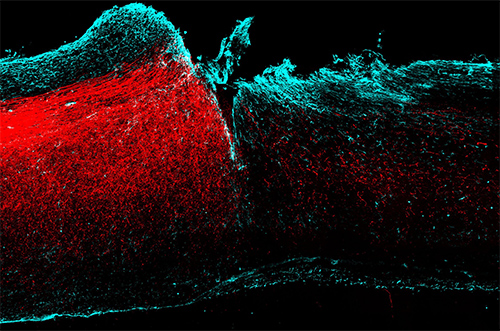
Axons from the motor cortex making new circuits in the injured spinal cord.
Nature Neuroscience/ Hollis et al.
Neurobiologists at UC San Diego have discovered how signals that orchestrate the construction of the nervous system also influence recovery after traumatic injury. They also found that manipulating these signals can enhance the return of function.
Most people who suffer traumatic injuries have incomplete lesions of neural circuits whose function can be partially restored from the reconfiguration of the spared circuits with rehabilitative training. But the mechanisms are not well understood.
In this week’s issue of Nature Neuroscience, biologists at UC San Diego report that removing the gene that encodes Ryk, a cell surface receptor for signaling proteins that control the wiring of the nervous system in development, enhances the ability of adult mice to remodel their neural circuits for the recovery of fine motor control after spinal cord injury.
“Our new study now provides the first genetic evidence that those signaling proteins, important in wiring the nervous system in development, have a profound influence on how central nervous system axons respond to spinal cord injury,” said Yimin Zou, a professor in the Neurobiology Section of UC San Diego’s Division of Biological Sciences, who headed the research team. “This suggests that many other guidance cues, in addition to these signaling proteins, may also play roles in adult spinal cord repair. This opens up new opportunities to apply what we’ve learned in nervous system development to treat paralysis in adulthood.”
These signaling proteins, or “Wnts,” play important roles in cell-cell communication. In the development of the nervous system, they determine what types of nerve cells young neurons eventually become and how they are spatially organized.
Zou and colleagues in his laboratory discovered more than a dozen years ago that Wnt protein gradients in the developing embryo also guide the growth of axons, the nerve fibers that connect the nerve cells into the highly organized network of neural circuits. They also found subsequently that Wnts and their receptors are not expressed, or are expressed at very low levels, in the adult spinal cord and the motor cortex in the brain. But in mice that underwent a spinal cord injury, they discovered that the Wnt proteins reappeared around the lesioned area in the spinal cord, while the receptor for the Wnt proteins, Ryk, appeared in the neurons of the motor cortex that project axons to the spinal cord to control body movement.
To elucidate the role of the Wnt proteins in spinal cord repair, the scientists developed a strain of mice that allowed them to remove Ryk from the motor cortex of the adult mouse.
“This way, the animals can develop normally,” said Zou. “And we can access the role of Ryk only in adulthood to see whether it inhibits axon regrowth after injury.”
Using this strain of mice and a normal control group, the scientists lesioned the dorsal column—the white matter tract within the spinal cord—at the C5 location of the spine to mimic a partial spinal cord injury in the mice. This severed their motor and sensory axons, but left the gray matter and the rest of the white matter within their spinal cords intact.
“Like people with a partial spinal cord injury, sensory and motor function in mice can recover partially with rehabilitative training,” said Zou. “We used a special task to test the ability of the animals to use the motor cortex to control skilled movement. Mice usually do not use their forepaw to grasp food pellets. However, they can be trained to learn this task, which depends on the command from the motor cortex. After training, we selectively injured the nerve fiber that sends commands for fine motor control (the corticospinal tract, which are the descending fibers in the dorsal column) and watch how fine motor control recovers with rehabilitative training.”
After training, the scientists found that their mice that lack Ryk recovered significantly better one month after injury and stay better for months to come.
“We found that manipulating Ryk will not only improve the results of functional recovery, but also speed up recovery,” said Zou. “If this can be achieved in humans, it will significantly improve the recovery and quality of life for individuals with partial spinal cord injuries.”
The researchers also generated a monoclonal antibody that blocked the function of the Wnt receptor, Ryk. This antibody significantly improved the recovery of fine motor skills in rats with the same spinal cord injury measured by the same reaching and grasping task.
“This monoclonal antibody could be a promising therapeutic tool not only for spinal cord injuries, but traumatic brain injury and stroke,” said Zou. “Another interesting finding in this new study is that maximal recovery from spinal cord injuries can only be achieved by combining molecular manipulation with rehabilitative training. This is important for designing therapeutic strategies.”
The other UC San Diego biologists involved in the study were Edmund Hollis, Ting Yu, Chin-Chin Lu, Ariela Haimovich, Kristine Tolentino, Alisha Richman and Maysam Pessian. Two other co-authors, Shih-Hsiu Wang and Alex Kolodkin, were from The Johns Hopkins School of Medicine.
The study was supported by grants from the National Institutes of Health (RO1 NS047484, R21 NS081738), Wings for Life Foundation and the International Foundation for Research in Paraplegia.
